
Written by Nancy Atkinson Since the 1890s, surface temperatures on Earth have risen faster in the Arctic than in other regions of the world. Usually, discussions on global warming tend to focus on greenhouse gases as the culprit for the trend. But new NASA research suggests about half the atmospheric warming measured in the Arctic is due to airborne particles called aerosols.
Aerosols are emitted by both natural and human sources. They can influence climate by reflecting or absorbing sunlight. The particles also affect climate by changing cloud properties, such as reflectivity. There is one type of aerosol that, according to the study, reductions rather than increases in its emissions seem to have promoted warming.
The research team, led by climate scientist Drew Shindell of the NASA Goddard Institute for Space Studies used a computer model to investigate how sensitive different regional climates are to changes in levels of carbon dioxide, ozone, and aerosols.
They found that Earth’s middle and high latitudes are particularly responsive to changes in aerosol levels. The model suggests aerosols likely account for 45 % or more of the warming measured in the Arctic since 1976.
Though there are several types of aerosols, previous research indicates two in particular, sulfates and black carbon, play leading roles in climate. Both are products of human activity. Sulfates, which come mainly from the burning of coal and oil, scatter sunlight and cool the air. Over the past three decades, the United States and European countries have passed clean-air laws that have halved sulfate emissions.
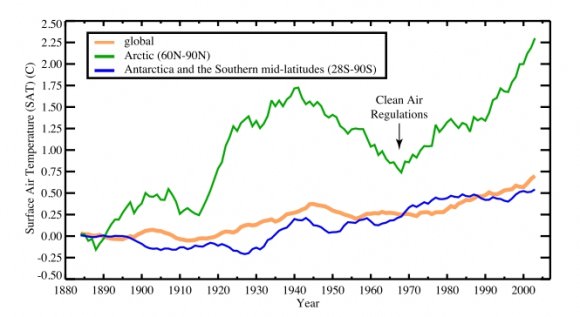
Since the 1890s, surface temperatures have risen faster in the Arctic than in other regions of the world. In part, these rapid changes could be due to changes in aerosol levels. Clean air regulations passed in the 1970s, for example, have likely accelerated warming by diminishing the cooling effect of sulfates. Credit: Drew Shindell, Goddard Institute for Space Studies
Researchers with the NOAA, the National Oceanic and Atmospheric Administration reported in the April 3 issue of the journal Geophysical Research Letters that Arctic summers may be ice-free in as few as 30 years.
The Arctic region has seen its surface air temperatures rise by 1.5 C (2.7 F) since the mid-1970s. In the Antarctic, surface air temperature has increased about 0.35 C (0.6 F). That makes sense, Shindell said, because the Arctic is near North America and Europe, highly industrialized regions that produce most of the world’s aerosols.
“In the mid-latitudes of the Northern Hemisphere and in the Arctic, the impact of aerosols is just as strong as that of the greenhouse gases,” said Shindell. “We will have very little leverage over climate in the next couple of decades if we’re just looking at carbon dioxide. If we want to try to stop the Arctic summer sea ice from melting completely over the next few decades, we’re much better off looking at aerosols and ozone.”
Aerosols tend to be short lived, staying in the atmosphere for just days or weeks, whereas greenhouses gases can persist for centuries. Atmospheric chemists thus think the climate may respond most quickly to changes in aerosol levels.
NASA’s upcoming Glory satellite is designed to enhance current aerosol measurement capabilities to help scientists reduce uncertainties about aerosols by measuring the distribution and properties of the particles.
Source: NASA
Filed under: Earth Observation, Environment

No comments:
Post a Comment